Energy Balances IV
Contents
Energy Balances IV#
This lecture continues with Energy Balance example problems.
import matplotlib.pyplot as plt
import numpy as np
import scipy.optimize as opt
from scipy.integrate import solve_ivp
from scipy.interpolate import interp1d
Energy Balances on Ideal Reactors#
Batch Reactor#
The material balance for species j in a well-mixed batch reactor is:
In cases where we have either an incompressible fluid or our reactor operates at constant pressure, the energy balance on a batch reactor is:
A few notation conventions: the bar over a property here means it is an intensive molar property for a species and it has units of “per mole”. \(\dot{Q}\) is the rate of heat exhange with the surroundings. It is given by:
CSTR#
The material balance for species j in a CSTR is:
If we have an incompressible fluid or a reactor operating at constant pressure, the energy balance on a CSTR is:
The rate of heat exchange is the same as in a batch reactor:
PFR#
The material balance for species j in a PFR is:
If we have an ideal gas or a reactor operating without a pressure drop, the energy balance for a PFR is:
For a PFR, we express the rate of heat transfer per unit volume, and it has a symbol \(\dot{q}\):
Example Problem 01#
(Fogler, Example 12.5)
This example considers non-isothermal operation of a PFR with multiple reactions. Specifically, the following two gas phase reactions:
Where both reactions have elementary rate laws. Pure A (\(C_{Af} = 0.1\) moles per liter) is fed to the reactor at a rate of 100 moles per second and a temperature of 150\(^\circ\)C. We additionally are provided the following information:
Note: reported rate constants were measured at 300K
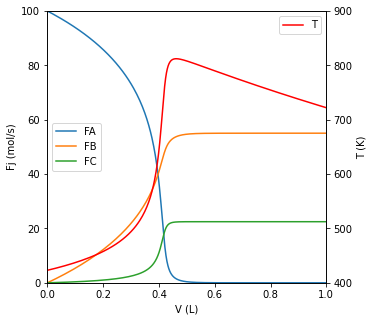
Assume this PFR has a total volume \(V = 1.0 \ \mathrm{L}\) ; plot the molar flowrates of all species as well as the temperature as a function of reactor volume.
Solution to Example Problem 01#
We are asked to plot the flowrate of each species and the temperature as a function of reactor volume. Clearly, we need to write material and energy balances.
For this system, we have 3 species, so we write 3 material balances:
We also write an energy balance on the reactor:
Now we just specify everything on the right hand side of those ODEs in terms of constants, the 4 dependent variables, or volume (our independent variable).
Production rates:
Rate expressions:
Concentration of A:
Use an equation of state to define Q:
Where R and P are both constant and expressed in appropriate units, and T is a state variable in the ODE system. We define total molar flowrate in terms of individual species:
Next, we need to define our rate constants as functions of temperature; we do so using a van’t Hoff type expression (since we are given rate constants measured at a specific temperature).
We also need to know the heats of reaction at the reaction temperature in order to complete the material balance. We know that, generally:
For both of these reactions, we find that \(\Delta C_p = 0\), so we conclude that heats of reaction are temperature independent here.
This is a non-isothermal reactor with heat exchange; we calculate the rate of heat exchange per unit volume (\(\dot{q}\)) as follows:
Where the temperature of the heat exchange fluid (\(T_a\)) and the product of the heat transfer coefficient (U) and the surface area-per-volume (a) are given in the problem statement.
That should be it. ODE system is fully specified and can be solved.
def P01(V, var):
#Fixed values from problem statement
Tf = 150 + 273 #K
Cf = 0.1 #mol/L
R = 0.08206 #L*atm/mol/K
Pf = Cf*R*Tf #atm
P = Pf #atm
k10 = 10 #1/s
k20 = 0.045 #L/mol/s
DH1 = -20000 #J/mol
DH2 = -120000 #J/mol
EAR1 = 4000 #K
EAR2 = 9000 #K
CPA = 90 #J/mol/K
CPB = 90 #J/mol/K
CPC = 180 #J/mol/K
Ua = 4000 #J/L/K/s
Ta = 100+273 #K
T0 = 300 #K
#Quantities changing as a function of reactor volume.
FA = var[0]
FB = var[1]
FC = var[2]
T = var[3]
FT = FA + FB + FC #mol/s
Q = FT*R*T/P #L/s
CA = FA/Q #mol/L
k1 = k10*np.exp(-EAR1*(1/T - 1/T0)) #1/s
k2 = k20*np.exp(-EAR2*(1/T - 1/T0)) #L/mol/s
r1 = k1*CA #mol/L/s
r2 = k2*CA**2 #mol/L/s
RA = -r1 - 2*r2 #mol/L/s
RB = r1 #mol/L/s
RC = r2 #mol/L/s
qdot = Ua*(Ta - T) #J/L/s
DT = (-1*(DH1*r1 + DH2*r2) + qdot)/(FA*CPA + FB*CPB + FC*CPC)
D1 = RA
D2 = RB
D3 = RC
D4 = DT
return [D1, D2, D3, D4]
FAf = 100 #mol/s
FBf = 0
FCf = 0
Tf = 150 + 273 #K
var0 = [FAf, FBf, FCf, Tf]
vspan = (0.0, 1.0) #L
ans = solve_ivp(P01, vspan, var0, atol = 1e-10, rtol = 1e-10)
V = ans.t
FA, FB, FC, T = ans.y
fig1, ax1 = plt.subplots(figsize = (5, 5))
ax2 = ax1.twinx()
ax1.plot(V, FA, label = 'FA')
ax1.plot(V, FB, label = 'FB')
ax1.plot(V, FC, label = 'FC')
ax2.plot(V, T, color = 'red', label = 'T')
ax1.set_xlim(0.0, 1.0)
ax1.set_ylim(0, 100)
ax2.set_ylim(400, 900)
ax1.set_xlabel('V (L)')
ax1.set_ylabel('Fj (mol/s)')
ax2.set_ylabel('T (K)')
ax1.legend(loc = 'center left')
ax2.legend(loc = 'upper right')
<matplotlib.legend.Legend at 0x205c7a7c910>